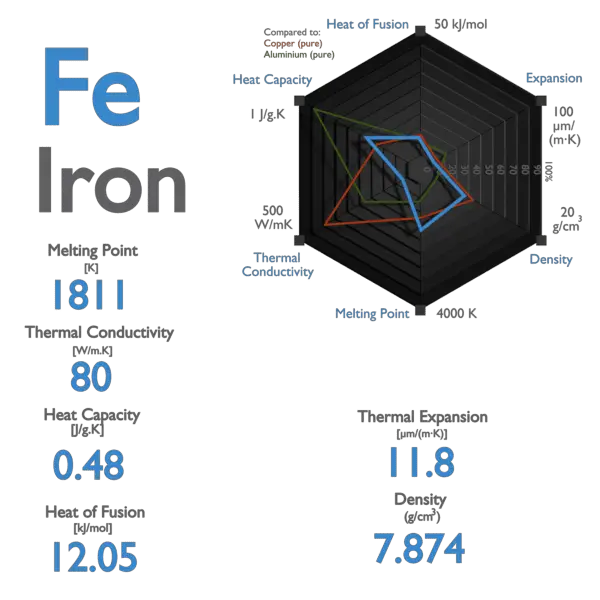
SOLVED: The specific heat capacity of iron is 0.45 J/g °C. How many joules of energy are needed to warm 1.96 g of iron from 20.00 °C to 29.0 °C? Determine the
Cheat calculations Physics Homework Help, Physics Assignments and Projects Help, Assignments Tutors online

Calculate the amount of heat required to raise the temperature of 5 g of iron from `25^(@)C \"to\" - YouTube
A 1.22-kg piece of iron at 126.5 °C is dropped into 981 g water at 22.1 °C. The temperature rises to 34.4 °C, what is the specific heat of iron, in J g-1°C-1? - Quora

Figure 3 from Thermodynamics of iron sulfides I. Heat capacity and thermodynamic properties of Fe9S10 at temperatures from 5 K to 740 K | Semantic Scholar
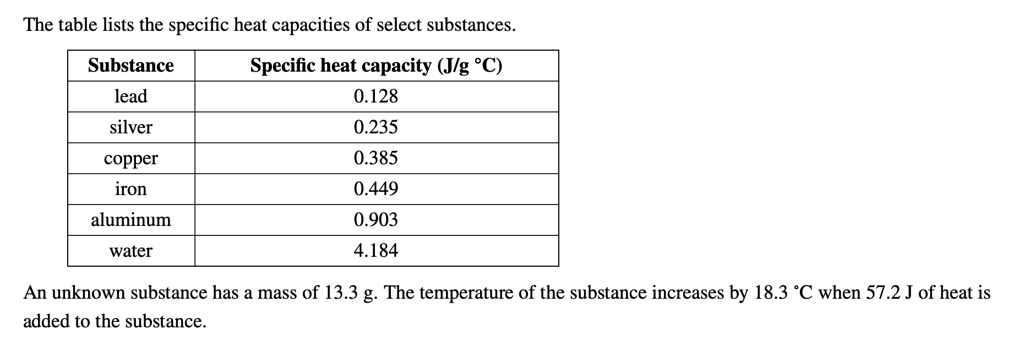
SOLVED: The table lists the specific heat capacities of select substances: Substance Specific heat capacity (J/g°C) lead 0.128 silver 0.235 copper 0.385 iron 0.449 aluminum 0.903 water 4.184 An unknown substance has
Why isn't the specific heat capacity of brass the average of the specific heat capacity of its components (which are copper and zinc)? Both copper and zinc have values of 376.8 J/(kg*K)







![Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram](https://www.researchgate.net/publication/332219094/figure/fig1/AS:744154090987520@1554431569138/Molar-heat-capacity-of-iron-Symbols-indicate-measurements-from-10.png)